Research
An introduction
Through evolution, nature combines twenty canonical amino acids to produce polymers of exquisite complexity. These combinations have resulted in a diverse set of peptides and proteins with diverse three-dimensional structures and function. This diversity however, is limited to evolutionary pressure. In the Rhys lab we are interested in going beyond what nature has explored. Using the same building blocks as nature we build and alter proteins for new applications, some of which are highlighted here.

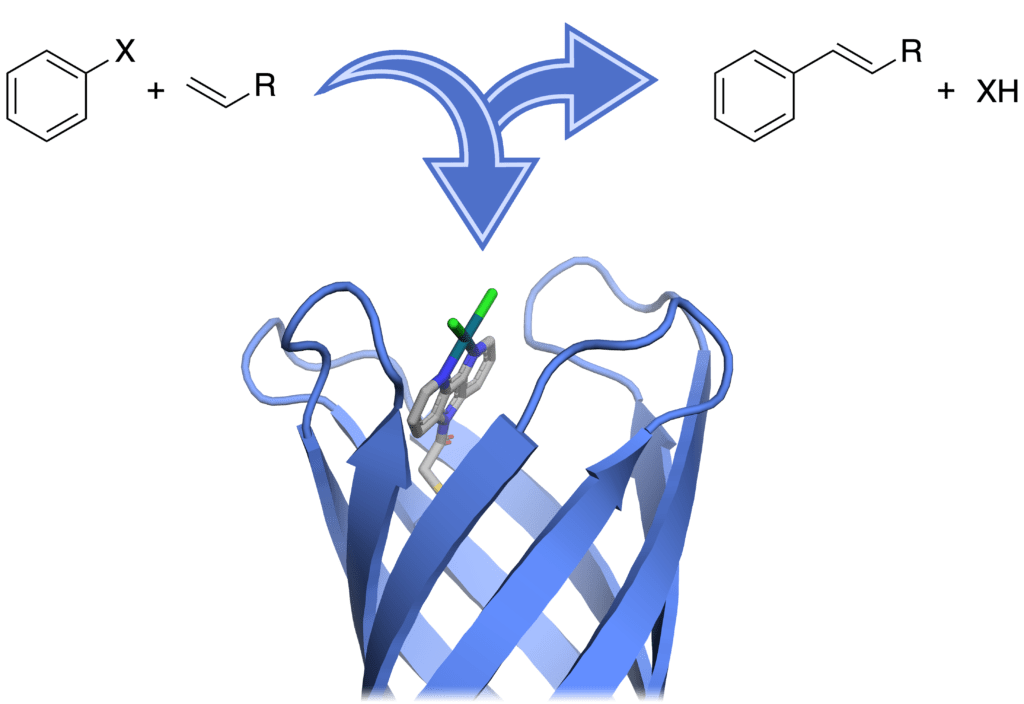
Artificial metalloenzymes
ArMs are proteins engineered to contain non-natural metal complexes. We are currently focusing on synthesising transition-metal complexes that will be bound to highly stable proteins and exploring cross-coupling reactions. Using these ArMs we will develop environmentally friendly alternatives to existing cross-coupling reactions, by catalysing reactions in water at near-ambient temperature using more earth-abundant metals. For current developments in ArMs research see the excellent overview by Jarvis and in-depth reviews by Klein et al. and Davis et al.
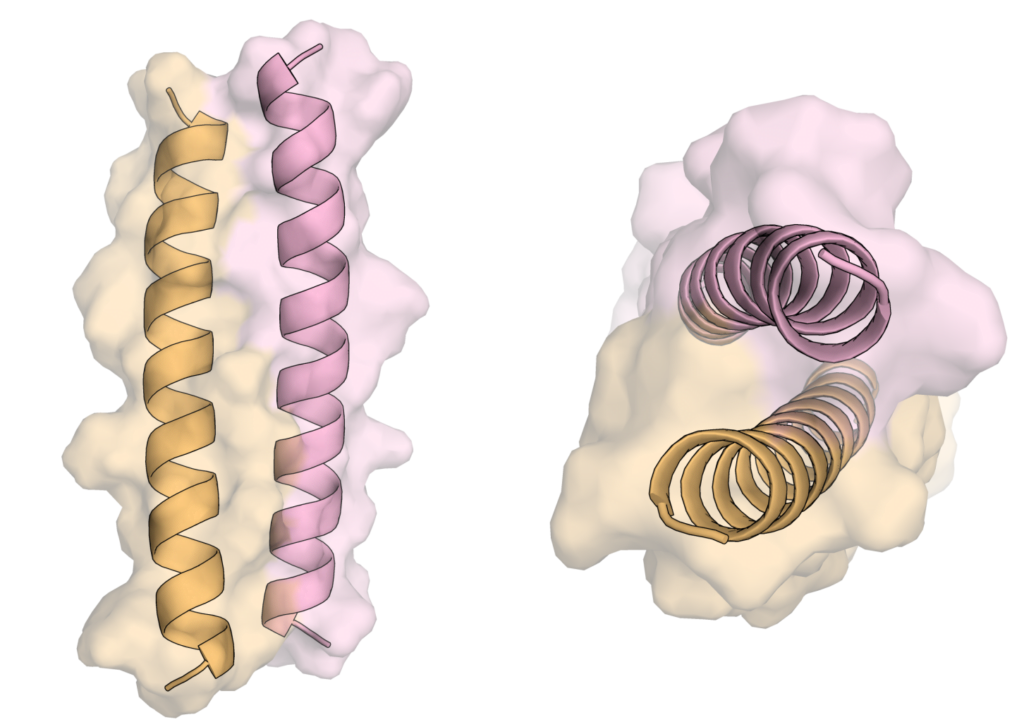
Coiled coils
CCs are a type of protein structure composed of two or more α helices that wrap around each other to form a specific interaction. This type of structure is found throughout nature serving several different functional roles. For example, in humans, CCs are a major component that gives strength to our nails and hair (α keratin), and in cells CCs regulate some of our genes (bZIP). Taking advantage of over 40 years of research into designing and engineering CCs, we are interested in developing CCs for new applications. For an overview of natural and designed CCs see review by Lupas et al., and subsections of reviews by Korendovych et al. and Woolfson.
Publications
For up-to-date publication list from the lab please visit Dr Rhys’ Google Scholar or ORCID pages. If you do not have subscription access to a particular manuscripts, get in contact with Guto via email with the subject “Manuscript access”.

